

because those electrons have similar average distances from the nucleus. All the wave functions that have the same value of n because those electrons have similar average distances from the nucleus. All wave functions that have the same value of n are said to constitute a principal shell. Add up the atomic masses of the Steps for. This means that electrons with higher values of n are easier to remove from an atom. Ionization potential of H atom is 13.59 electron volts 1 L of gas at 0 C weighs. (This formula only means the hydrogen peroxide molecule contains equal numbers of hydrogen and oxygen atoms. A negatively charged electron that is, on average, closer to the positively charged nucleus is attracted to the nucleus more strongly than an electron that is farther out in space. Recall that two hydrogen atoms bind to make.
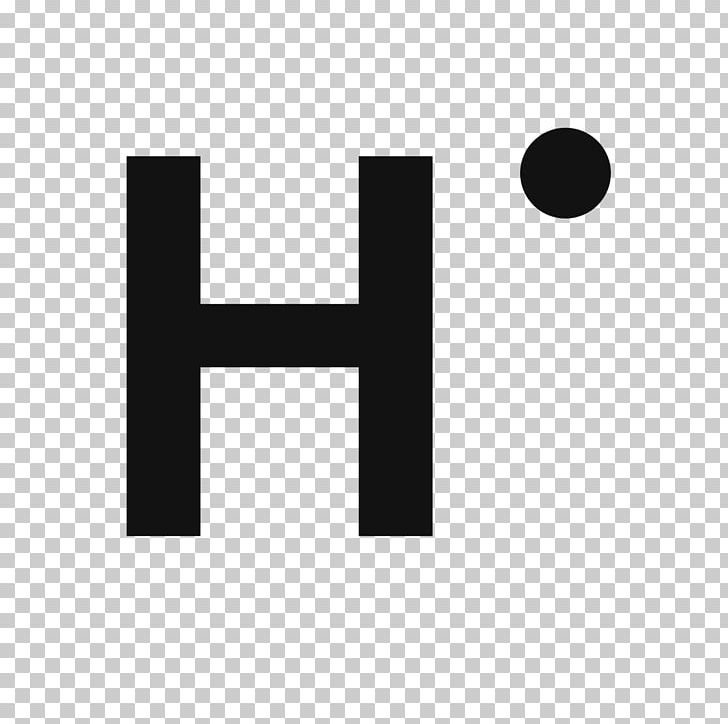
So, because the mass of hydrogen is close to 1 amu (atomic mass unit),1 gram of hydrogen contains about that same huge number of atoms.\]Īs\( \)n increases for a given atom, so does the average distance of an electron from the nucleus. However, one mole of hydrogen atoms has a mass of 1 gram while one MOLE of hydrogen molecules has a mass of 2 grams. Bohr model of the Hydrogen Atom The atoms are generally made up of protons, neutrons and electrons. The first electron in helium has four quantum numbers of the first electron hydrogen. When the quantum number l0 there is only one value for m, which would be zero. Hydrogen has an atomic mass of 1.01 atomic mass units. The atomic molar mass of the hydrogen is 1.0078 u. Atomic mass of Hydrogen 1 u Atomic mass of Oxygen 16 u Relative molecular mass of water (H2O) 2 × Atomic Mass of Hydrogen + 1 × Atomic Mass of Oxygen 2 × 1 + 1 × 16 18 u Example 3. That means it is the same as the number of hydrogen. For every mole, there are 6.02*10 23 atoms, so in 1 gram of hydrogen there are 6.02*10 23 hydrogen atoms (in scientific notation) this is equal to 602000000000000000000000 hydrogen atoms. On average, each gram of matter has around 1024 protons, according to Fermilab, a national laboratory for particle physics in Illinois. Multiply and round the answer to 5 significant figures.īecause the molecular mass of Hydrogen is 1 gram/mole, there is 1 mole of hydrogen in 1 gram of hydrogen atoms. The original standard of atomic weight, established in the 19th century, was hydrogen, with a value of 1. Multiply both sides of the equation by 1.0 g H to isolate the variable x on the right side.ġ.0000 g H * (6.022 x 10 23 atoms H / 1.0079 g H) = x atoms HĦ.0221 x 10 23 atoms H / 1.0079 = x atoms H So, if there is 1.0079 grams of hydrogen per mole, then you can find out the number of hydrogen atoms in 1.0000 grams using the following proportion:Ħ.0221 x 10 23 atoms H / 1.0079 g H = x atoms H / 1.0000 g H The atomic weight of hydrogen is 1.0079, which means that one mole of hydrogen atoms (or 6.0221 x 10 23 atoms of hydrogen) has a mass of 1.0079 grams. These isotopes with an additional neutron add a tiny amount to hydrogens average atomic weight. An even smaller proportion of hydrogen atoms are the isotope tritium, or hydrogen-3, which has one proton and two neutrons. Hydrogen has an atomic weight of 1.0079 rather than 1.0000 because a small proportion of hydrogen atoms are the isotope deuterium, or hydrogen-2, which has one proton and one neutron rather than just one proton. The hydrogen atom consists of two particles, the proton and the electron, interacting via the Coulomb potential V(r1r2)e2/r, where as usual rr1r2. How many hydrogen atoms would you find in 1g of hydrogens?Ī mole (Avogadros number) is the number that is equivalent to the number of atoms in 12 grams of pure carbon-12, which is always 6.022 x 10 23 atoms.


 0 kommentar(er)
0 kommentar(er)
